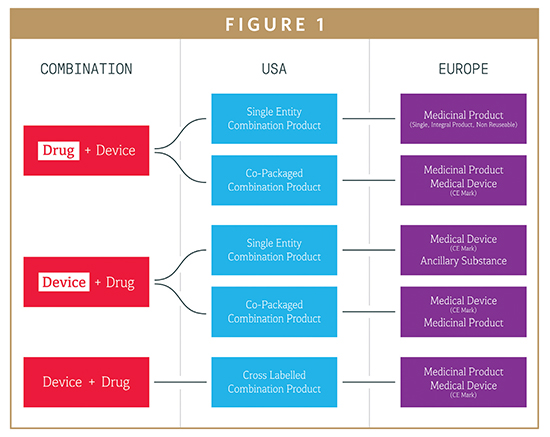
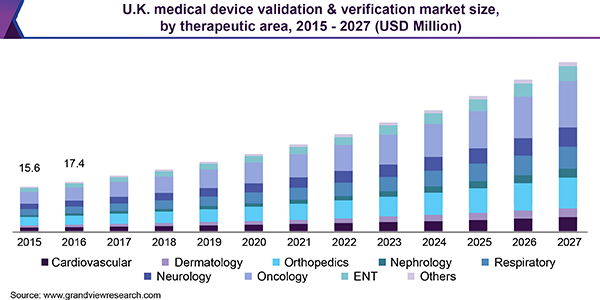
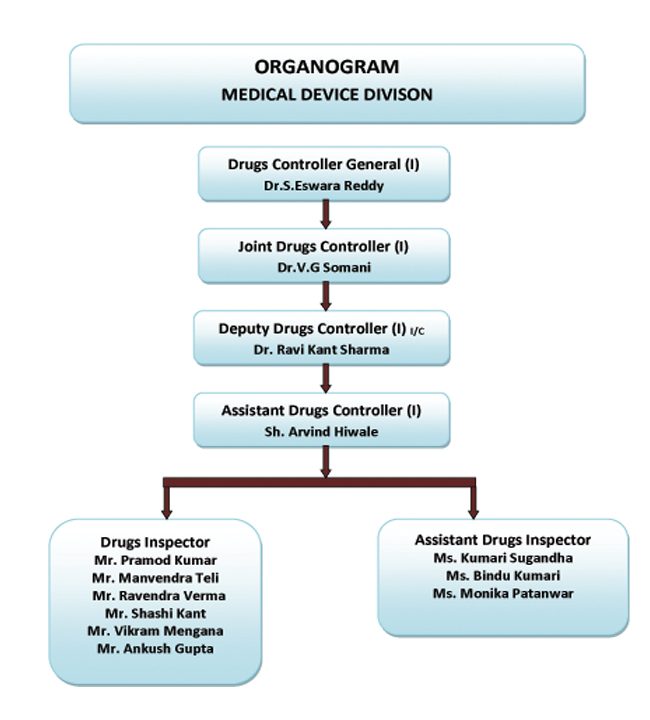
Although the united kingdom will have officially left the european union by the end of march 2019 the country is continuing to regulate medical devices using the mdr and ivdr regulations which were introduced in may 2017.
Medical device regulations 2017 uk.
Regulation eu 2017 745 of the european parliament and of the council show full title.
Both regulations entered into force in may 2017 and have a staggered transitional period.
Entering into force on 25 may 2017 the three and five year transition periods also known as implementation are now underway.
The new guide should prove useful for ce mark certificate holders has they prepare for full compliance with the medical devices regulation mdr 2017 745 and in vitro diagnostic regulation ivdr 2017 746.
However elements of both new devices regulations have applied directly in uk law since may 2017 meaning medical devices including ivds can now be legally placed on the uk market if they are in.
A new interactive online guide from the uk mhra provides high level overviews of new european medical device and ivd regulations.
Added a link to guidance for manufacturers who don t design or manufacture devices but place their names.
These directives are transposed into uk law in the medical devices regulations 2002 si 2002 no 618 as amended uk mdr 2002.
Medical devices regulations 238 kb pdf full document.
Regulation 2017 745 on medical devices eu mdr.
Regulation eu 2017 745 of the european parliament and of the council of 5 april 2017 on medical devices amending directive 2001 83 ec regulation ec no 178 2002 and regulation ec no 1223 2009 and repealing council directives 90 385 eec and 93 42 eec text with eea relevance.
I legislative acts regul ations regul ation eu 2017 745 of the european parliament and of the council of 5 apr il 2017 on medical devices amending directive 2001 83 ec regulation ec no 178 2002 and.
The new regulations will apply across eu member states from 26 may.